Fluorescence Spectroscopy
Definition: spectroscopy which is based on the analysis of fluorescence light
More general terms: optical spectroscopy
German: Fluoreszenzspektroskopie
Categories: optical metrology, methods
How to cite the article; suggest additional literature
Author: Dr. Rüdiger Paschotta
Fluorescence spectroscopy denotes a class of spectroscopy methods which are based on the analysis of fluorescence light, particularly concerning the emission spectrum. Properties of the fluorescence are frequently used to identify substances, often including their concentrations, and in other cases properties of a medium which influence details of the fluorescence.
The term fluorescence spectrometry is sometimes used instead of spectroscopy in order to emphasize that certain quantities are measured in a quantitative manner.
In contrast to absorption spectroscopy, one usually does not measure what happens to the applied excitation light (e.g. its absorbance), but observes only the properties of the fluorescent emission.
Operation Principles
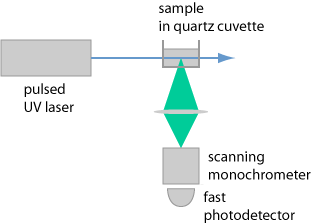
Fluorescence is actively excited by irradiating a sample with light from some light source which is part of the fluorometer (or fluorimeter). (In many cases, one uses ultraviolet light.) Further, there is some kind of photodetector, which may be combined with a tunable monochromator (or sometimes only a few simple optical filters) for spectral analysis. Figure 2 shows an example for a fluorometer setup.
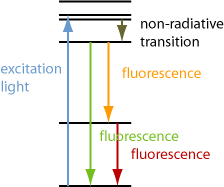
Some fluorophore in the sample usually absorbs the incident excitation light via linear absorption (with one photon per absorption process), but there are also cases where nonlinear absorption at high optical intensities is utilized, for example two-photon absorption or even multiphoton absorption, i.e., with two or more photons per excitation process. The absorption causes the excitation of atoms, ions or molecules to certain excited electronic states. Frequently, the excited objects first undergo some non-radiative transitions to lower excited states (see Figure 1). Finally, with some probability there will be emission of a photon from the excited state either to the electronic ground state or to another excited state, in the latter case with a correspondingly reduced photon energy and possibly followed by the emission of one or more additional photons. There are also cases with resonance fluorescence, which do not involve additional electronic transitions, and the emission wavelengths are close to the excitation wavelength. Part of the fluorescence may be quenched.
In free molecules, for example in gas spectroscopy, the involved excited states can involve vibrational and rotational states. In case of solid materials, interactions with neighbored atoms can substantially modify the produced fluorescence. Similarly, there are solvent interactions in liquids.
Some kind of samples contain natural kinds of fluorophores, which can be parts of certain larger molecules or separate fluorescent molecules. For example, tyrosine and tryptophan occur in biological samples. In other cases, one adds a fluorophore in the form of some fluorescent dye (frequently aromatic compounds such as fluorescein), which can help to reveal certain properties of the sample. For example, certain dyes preferentially get attached to certain structures and can therefore act as markers for those. Such methods are particularly used in fluorescence microscopy e.g. for biological research, where certain structures within biological cells can be marked.
In most cases, the excitation wavelength (or a broadband excitation spectrum) stays fixed. However, there are also methods where one scans the excitation wavelength through a certain range and sometimes detects only the overall optical power of the fluorescence (without spectral analysis). One then focuses on the details of the excitation mechanism rather than on the details of light emission. Maximum flexibility is obtained when combining a variable excitation wavelength with full spectral analysis of the fluorescence.
The excitation light can be a continuous-wave source or a pulsed source:
- For continuous excitation, one may analyze the fluorescence light mostly concerning its optical spectrum, and sometimes also concerning the polarization.
- For pulsed excitation, one can also measure the fluorescence lifetime (decay time) – either of the complete light or of specific spectral components. The fluorescence lifetime (e.g. in the nanosecond or microsecond region) can convey additional spectroscopic information. For example, fluorescence of certain ions in a solid may be quenched when they are close to certain crystal defects or impurities.
Instruments for fluorescence spectroscopy can be called spectrofluorometers. (There are also simpler fluorometers or fluorimeters without spectral resolution.) Apart from high-performance instruments for use in laboratories, there are also more compact ones, even in the form of handheld battery-powered devices.
The excitation and/or the light detection may be restricted to a small volume on the sample. Fluorescence spectroscopy measured with a high spatial resolution can be called fluorescence microscopy.
Light Sources for Fluorescence Spectroscopy
Depending on the chosen method, different kinds of light sources can be used for fluorescence spectroscopy:
- Simple light sources such as certain gas discharge lamps (e.g. mercury vapor lamps) can be used for generating ultraviolet light, either with continuous or pulsed emission. One then often requires some kind of optical bandpass filter for restricting the spectral range. There are also sources with an excitation monochromator with which one scans through a certain range during the measurements.
- In some cases, even light emitting diodes (LEDs) are sufficient. That allows for cheap and compact solutions, particularly for portable instruments.
- Lasers of different kinds are particularly powerful excitation sources, since one can address a wide spectral range, including the ultraviolet, which is then usually generated with frequency doubling in a nonlinear crystal. It may be of interest to do the excitation with light having a narrow optical bandwidth (linewidth). Further, with pulsed lasers one can easily obtain short pulses with durations of a few nanoseconds, or even picoseconds or femtoseconds.
- For excitation with widely variable wavelengths, one may use tunable lasers or optical parametric oscillators (OPOs), or a broadband source in conjunction with an excitation monochromator as mentioned above. In the latter case, the available excitation intensities are of course far smaller than with lasers.
If the used light source cannot produce light with a precisely constant optical power or pulse energy, one may also measure its output with an additional photodetector and use its output signal to eliminate power fluctuations from the results of the fluorescence measurements.
Photodetectors
Different kinds of photodetectors can be used, depending on the requirements. For instruments with continuous-wave excitation and a scanning monochromator, one simply requires a photodetector with high sensitivity in the relevant spectral range – for example, some type of photodiode. In conjunction with a dual monochromator for optimum suppression of stray light, one requires a tentatively even higher detector sensitivity.
Another possibility is to use a kind of spectrograph containing a diffraction grating and a linear photodiode array or other kind of photodetector with spatial resolution. When using a two-dimensional sensor, such as otherwise used as an image sensor, one may achieve one-dimensional spatial resolution in combination with spectral resolution.
For fluorescence lifetime measurements, one requires a fast response (high bandwidth) in addition to the high sensitivity. This narrows the choice of suitable detectors; one often uses photomultipliers or avalanche photodiodes. There are also devices containing a microchannel plate.
Various Issues
One typically tries to eliminate any direct effect of excitation light on the detector, e.g. by spectral discrimination, possibly also with other methods, e.g. by detection in a direction where little excitation light is expected, e.g. perpendicular to an excitation beam in a transparent sample. Particularly in cases where the fluorescence is much weaker than the excitation light, or when the sample is strongly scattering the excitation light, strong suppression of excitation light in the detection system is required. For example, one may need to use a double monochromator.
For efficient light collection from the sample e.g. to the input slit of a monochromator, one can use a cylindrical lens.
The obtained fluorescence spectra may be modified by further interactions within the sample, for example by wavelength-dependent absorption. Such effects can be minimized by using thin samples.
In addition to fluorescence light, there may also be light arising from Raman scattering, which also exhibits some Stokes shift.
In some cases, the ultraviolet excitation light causes degradation (e.g. by photodecomposition) of the sample. Highly sensitive detection is then particularly desirable, since it allows one to reduce the exposure time.
Sometimes, one needs to monitor not only the amount of fluorescence from a certain fluorophore, but precisely detect minor changes of the emission spectrum, for example due to subtle interactions in the sample. A particularly high spectral resolution and low detection noise may be required in such cases.
Applications
Fluorescence spectroscopy is often used in chemistry, biology and environmental monitoring. Some examples:
- Industrial products, for example in the petroleum industry, or pharmaceutical products can be monitored with detection of fluorescence, for example in order to detect impurities. One may identify substances and measure their concentrations.
- Similar applications are in environment monitoring, e.g. on water samples or in air.
- Various bacteria, viruses, fungi and parasites, causing dangerous infections, can be detected and distinguished through fluorescence spectroscopy. Also, biomedical research on antibiotic sensitivity of microorganisms can profit from such methods.
- Another application is cancer diagnostics in human tissues. For example, one may infiltrate a sample with a substance which is preferentially accumulated in tumors and can then be detected through laser-induced fluorescence.
- As already mentioned above, fluorescence markers are widely used in fluorescence microscopy for biological research.
Time-resolved fluorescence spectroscopy can be used to gain substantial additional information and is thus interesting for certain areas of fundamental scientific research.
Suppliers
The RP Photonics Buyer's Guide contains 14 suppliers for fluorescence spectroscopy equipment. Among them:


NKT Photonics
Our supercontinuum lasers with built-in pulse picker in combination with our tunable SuperK VARIA filter is an extremely useful excitation source for time-resolved spectroscopy experiments. With its broad range it replaces solutions based on combining several lasers. It also eliminates time-consuming hardware modifications and alignments that would normally be required while switching excitation sources. The pulse picker can reduce the repetition rate to secure enough time for the photo-excited materials to reach their ground state before the next photoexcitation-fluorescence cycle is initiated.
Questions and Comments from Users
Here you can submit questions and comments. As far as they get accepted by the author, they will appear above this paragraph together with the author’s answer. The author will decide on acceptance based on certain criteria. Essentially, the issue must be of sufficiently broad interest.
Please do not enter personal data here; we would otherwise delete it soon. (See also our privacy declaration.) If you wish to receive personal feedback or consultancy from the author, please contact him e.g. via e-mail.
By submitting the information, you give your consent to the potential publication of your inputs on our website according to our rules. (If you later retract your consent, we will delete those inputs.) As your inputs are first reviewed by the author, they may be published with some delay.
See also: fluorescence, spectroscopy, fluorescence microscopy
and other articles in the categories optical metrology, methods
 |


If you like this page, please share the link with your friends and colleagues, e.g. via social media:
These sharing buttons are implemented in a privacy-friendly way!